Page 214 - Demo
P. 214
Safety Reference
Preparation of Simple Inorganic Salt Solutions, continued
1-800-452-1261
flinnsci.com
212
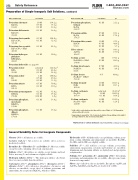
Name / Formula / F.W.
Potassium chromate K2CrO4
194.21
Potassium dichromate K2Cr2O7
294.22
Potassium ferricyanide K3Fe(CN)6
329.26
Potassium ferrocyanide K4Fe(CN)6 • 3H2O 422.41
Potassium hydrogen phthalate
KHC8H4O4 204.23
Concentration
1.0 M 0.5 M 0.1 M
0.1 M
0.5 M 0.1 M
0.1 M 0.1 M
g/L
194.2 97.1 19.4
Name / Formula / F.W.
Potassium phosphate, tribasic
K3PO4 212.27
Potassium sulfate K2SO4
174.27
Potassium thiocyanate
KSCN 97.18
Silver nitrate AgNO3
169.87
Sodium acetate NaC2H3O2 • 3H2O 136.08
Sodium bicarbonate NaHCO3
84.01
Sodium borate Na2B4O7 • 10H2O 381.42
Sodium bromide
NaBr 102.90
Sodium carbonate Na2CO3
105.99
Sodium carbonate Na2CO3 • H2O 124.00
Concentration g/L
Potassium hydroxide see page 86
Potassium iodate KIO3
214.01
Potassium iodide
Kl 166.01
Potassium nitrate KNO3
101.11
Potassium permanganate KMnO4
158.04
Potassium phosphate, monobasic
KH2PO4
136.09
Potassium phosphate, dibasic
K2HPO4 174.18
saturated 0.2 M 0.1 M
1M 0.5 M 0.2 M
0.5 M 0.1 M
0.2 M
0.1 M 0.01 M
0.1 M
0.1 M
214.0 g† 42.8 g 21.4 g
166.0 g 83.0 g 33.2 g
50.6 g 10.1 g
31.6 g 15.8 g 1.6 g
29.4 g
164.6 g 32.9 g
42.2 g
20.4
g
13.6
g
17.4 g
*Add solid to acid solution, stir, then add to water. Dilute to 1 L. Remember, always add acid to water.
†Approximate amount for 1 L of saturated solution. Keep adding solute until it no longer dissolves; stir for 1 hour, then filter.
g g g
0.1 M
0.5 M 0.1 M
1.0 M 0.5 M 0.1 M
0.5 M 0.1 M
1M 0.5 M
0.5 M 0.1 M
4%
1.0 M 0.1 M
saturated 1.0 M 0.1 M
1.0 M 0.1 M
21.2 g
87.1 g 17.4 g
97.2 g 48.6 g 9.7 g
84.9 g 17.0 g
136.1 g 68.0 g
42.0 g 8.4 g
40.0 g
102.9 g 10.3 g
214.0 g† 106.0 g 10.6 g
124.0 g 12.4 g
PREPARATION OF SIMPLE INORGANIC SALT SOLUTIONS continued on next page.
General Solubility Rules for Inorganic Compounds
Nitrates (NO3–): All nitrates are soluble.
Acetates (C2H3O2–): All acetates are soluble; silver acetate is
moderately soluble.
Bromides (Br–) Chlorides (Cl–) and Iodides (I–): Most are soluble except for salts containing silver, lead, and mercury.
Sulfates (SO42–): All sulfates are soluble except barium and lead. Silver, mercury(I), and calcium are slightly soluble.
Hydrogen sulfates (HSO4–) : The hydrogen sulfates (aka bisul- fates) are more soluble than the sulfates.
Carbonates (CO32–), phosphates (PO43–), chromates (CrO42–), silicates (SiO42–): All carbonates, phosphates, chromates, and silicates are insoluble, except those of sodium, potassium, and ammonium. An exception is MgCrO4, which is soluble.
Hydroxides (OH–): All hydroxides (except lithium, sodium, potas- sium, cesium, rubidium, and ammonium) are insoluble; Ba(OH)2, Ca(OH)2 and Sr(OH)2 are slightly soluble.
Sulfides (S2–): All sulfides (except sodium, potassium, ammonium, magnesium, calcium and barium) are insoluble. Aluminum and chromium sulfides are hydrolyzed and precipitate as hydroxides.
Sodium (Na+), potassium (K+), ammonium (NH4+): All sodium, potassium, and ammonium salts are soluble. (Except some transi- tion metal compounds.)
Silver (Ag+): All silver salts are insoluble. Exceptions: AgNO3 and AgClO4; AgC2H3O2 and Ag2SO4 are moderately soluble.